
Dalton: Created modern atomic theory. All atoms are hooked together, and form specific ratios. Did not realize that atoms are made up of different particles

Thomson: Discovered electron with cathode ray tube. Applied a magnetic force, and noticed that the ray deviated, due to opposing magnetism. Also created a new idea of appearance, titled plum pudding formation.

Rutherford: Fired alpha particles through gold, some rays deviated from center, while others bounced right back. This was because of the positively charged nucleus. He also realized that a lot of the volume of the atom is empty space.
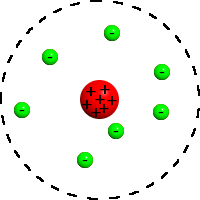
Bohr: Worked in Rutherford's lab, and realized if the charge on the inside was positive, that the negative and positive charges would attract. Created the Bohr model on the assumption that electrons followed specific paths.

Schroedinger: claimed that electrons weren't particles but were in fact waves.

Chadwick: Discovered the neutron by disintegrating atoms through bombardment of alpha particles. First to create artificial nuclear reaction
Heisenberg: Confirmed both of Bohr and Schroedinger's hypothesis. The electron is indeed a particle, that travels in a wave like motion, which is described by quantum theory.
Science instruments play an important role in the research laboratory. Good science lab equipment can make a success of your experiments.Brix refractometer
ReplyDeleteReally appreciate this post. It’s hard to sort the good from the bad sometimes, but I think you’ve nailed it!
DeleteLink - http://dingyichenchemistry.blogspot.in/2013/01/safety-in-laboratory.html